Chuck52
Veteran Member
Leather treatment is "related", isn't it?
A while back I bought some Kiwi Wet Pruf at Wally World to use on
my shoes. I was really looking for some mink oil or the like, but
the Kiwi stuff was all they had in the shoe department, and I figured
it would do. It's a yellow grease/wax stuff that is supposed to
water proof in about the same way mink oil does. I used it once
without noticing anything unusual. Saturday, I was applying another
coat and the sun shone through the window at just the right angle to
hit the can of goop. Sparkles! There were shiny crystals all over
the surface of the stuff where I hadn't yet dug into it.
You have to understand, crystals are my life. Crystals pay the bills. I
have a lab full of equipment to examine crystals. So....off to the
lab with the can of goop. Now, I was really hoping the crystals
were of some component of the goop that might be interesting, like one
of the greasy things that do the water proofing. Such molecules don't
like to crystallize, and I was thinking maybe I had lucked onto a really
interesting crystalline material. As soon as I got the crystals
under the scope and poked them with a needle, I knew the Nobel committee
wouldn't be looking me up for this little project. The crystals were
way too pretty and hard. If a miracle had happened and one of the
greasy components had crystallized, the crystals would have been
soft, at best. These little suckers acted more like sugar crystals.
Ah, well. Might as well see what they are.
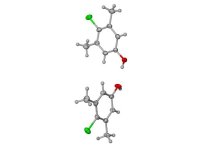
The structure is attached. Gray atoms are carbon and hydrogen. Red
is oxygen. Green is chlorine. The stuff is 4-chloro-3,5-dimethyl-
phenol, also called chloroxylenol. It's used for mildew prevention,
so it makes sense that it is included in this leather treatment. So,
why am I telling you this? Well, couple of reasons. One is that
there are no ingredients listed on the container of Wet Pruf. This
particular ingredient is not something a casual user of this product
might expect to be there. It is a fungicide, and in my experience,
fungicides are not good things to eat. Maybe you're neater about these
things than I am, but when I waterproof my shoes I also usually water-
proof my fingers. Thinking it's just grease, I'd probably wipe my hands
before eating that donut, but I wouldn't think much about it. Turns
out this stuff is reasonably water soluble, so a good washing might get
it off your hands, though you gotta wonder how much good it would then
do your leather! Anyway, I know some of my TBN bretheren are more
chemophobic than I, and might want to know. The other reason is that
I can't publish this structure because some guy already did it in 1995.
Plus, I've been jealous of all you guys who have neat pictures of
projects and stuff.
Chuck
A while back I bought some Kiwi Wet Pruf at Wally World to use on
my shoes. I was really looking for some mink oil or the like, but
the Kiwi stuff was all they had in the shoe department, and I figured
it would do. It's a yellow grease/wax stuff that is supposed to
water proof in about the same way mink oil does. I used it once
without noticing anything unusual. Saturday, I was applying another
coat and the sun shone through the window at just the right angle to
hit the can of goop. Sparkles! There were shiny crystals all over
the surface of the stuff where I hadn't yet dug into it.
You have to understand, crystals are my life. Crystals pay the bills. I
have a lab full of equipment to examine crystals. So....off to the
lab with the can of goop. Now, I was really hoping the crystals
were of some component of the goop that might be interesting, like one
of the greasy things that do the water proofing. Such molecules don't
like to crystallize, and I was thinking maybe I had lucked onto a really
interesting crystalline material. As soon as I got the crystals
under the scope and poked them with a needle, I knew the Nobel committee
wouldn't be looking me up for this little project. The crystals were
way too pretty and hard. If a miracle had happened and one of the
greasy components had crystallized, the crystals would have been
soft, at best. These little suckers acted more like sugar crystals.
Ah, well. Might as well see what they are.
The structure is attached. Gray atoms are carbon and hydrogen. Red
is oxygen. Green is chlorine. The stuff is 4-chloro-3,5-dimethyl-
phenol, also called chloroxylenol. It's used for mildew prevention,
so it makes sense that it is included in this leather treatment. So,
why am I telling you this? Well, couple of reasons. One is that
there are no ingredients listed on the container of Wet Pruf. This
particular ingredient is not something a casual user of this product
might expect to be there. It is a fungicide, and in my experience,
fungicides are not good things to eat. Maybe you're neater about these
things than I am, but when I waterproof my shoes I also usually water-
proof my fingers. Thinking it's just grease, I'd probably wipe my hands
before eating that donut, but I wouldn't think much about it. Turns
out this stuff is reasonably water soluble, so a good washing might get
it off your hands, though you gotta wonder how much good it would then
do your leather! Anyway, I know some of my TBN bretheren are more
chemophobic than I, and might want to know. The other reason is that
I can't publish this structure because some guy already did it in 1995.
Plus, I've been jealous of all you guys who have neat pictures of
projects and stuff.
Chuck